Start Stop Car Battery: Lead-acid Battery vs. Lithium-ion Battery
In 1859, French physician Gaston Planté pioneered the commercial use of lead acid — the first rechargeable battery. In recent years, lead-acid batteries have been optimized in numerous applications for its reliability and inexpensive cost-per-watt base but there remain certain new shortcomings facing the lead-acid battery manufacturers —an increase in the amount of faulty modes.
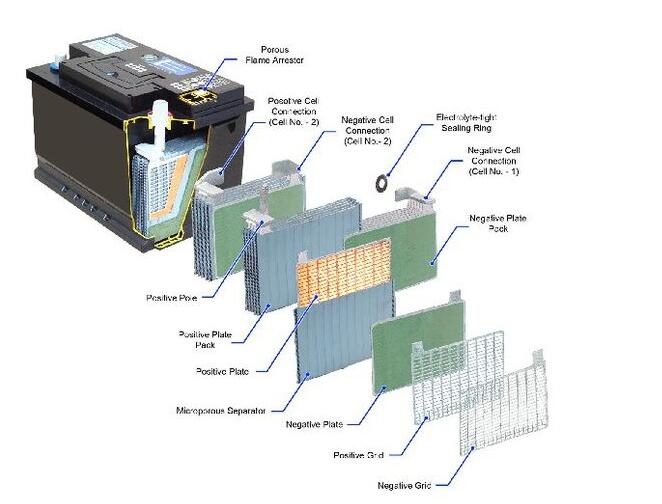
Image 1. Components of Lead Acid Battery (Source thebatteryclinic.co.nz)
Types of Lead-acid Batteries
●Batteries with flooded or excess electrolyte.
●Low-maintenance lead-acid batteries with a large excess of electrolyte.
●Batteries with immobilized electrolyte and a pressure-sensitive valve usually referred to as absorptive glass-microfibre (AGM) valve-regulated lead-acid (VRLA) batteries.
Advantage | Disadvantages |
●Inexpensive and simple to manufacture; low cost per watt-hour ●Low self-discharge; lowest among rechargeable batteries ●High specific power, capable of high discharge currents ●Good low and high temperature performance | ●Low specific energy; poor weight-to-energy ratio ●Slow charge; fully saturated charge takes 14-16 hours ●Must be stored in charged condition to prevent sulfation ●Limited cycle life; repeated deep-cycling reduces battery life Suggested reading:Jump Starter, the Game-Changer in Vehicle Emergencies What are the key advantages of using Rockchip SOM? How Vertiv Liebert UPS Works for Continuous Power Protection? What does a capacitor do to voltage? Demystifying Types and Applications of Resistors The Importance of Fully-Functional Traffic Lights - HDFC Ergo What is the difference between RF cable and coaxial cable? ●Flooded version requires watering ●Transportation restrictions on the flooded type ●Not environmentally friendly |
Table 1. Advantages and Limitations of Lead Acid Batteries (Retrieved from Battery University)
Overview of Lithium-ion Batteries
(Read also: The Best Start-stop Car Battery)
Lithium-ion began to surface in the 1970s, yet these immediate batteries are not rechargeable. Thus in the 1980s, rechargeable versions were manufactured. Nowadays, it's the most common battery in commercial use. Lithium is lightweight in contrast to other metals and the highest density per kilogram. Therefore, lithium is the most ideal for batteries since it contains significant amounts of energy and has the greatest electrochemical potential.
How do Lithium-ion Batteries work?
Inside a Lithium-ion battery there is a negative and positive electrode. The cathode is built from lithium cobalt oxide (LiCoO2). The anode is reared of carbon and both anode and cathodes are suppressed in an electrolyte. The battery is equipped with a separator between cathode and anode then the Lithium-ions from the cathode roams through the electrotype and separator to the anode.
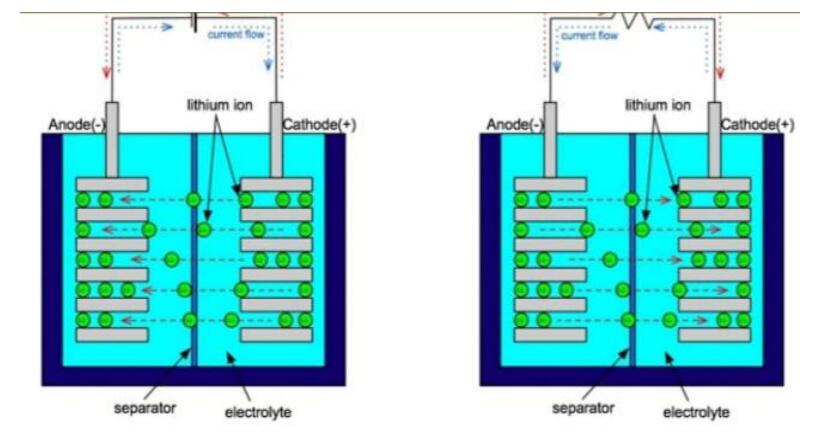
Image 2. Retrieved from physicsandsocietybc.wordpress.com
HOUNY has specifically developed and launched a series of car start-stop batteries that use automotive-grade lithium iron phosphate batteries and a built-in intelligent battery management system (BMS).
For more inquiries:
Website: www.hounypower.com
Phone Chargers: Powering Your Devices Anytime
Understanding Deep Cycle Battery Packs
Choosing the Right Wire Wound Resistor for Your Electronic Projects
Eye Bolts: The Reliable Workhorses of Various Industries
Advantages of Vertiv Liebert UPS Rectifier Cabinets
The Advantages of Stamp Hole Core Board SOMs
The Advantages of Lithium-ion Golf Cart Batteries